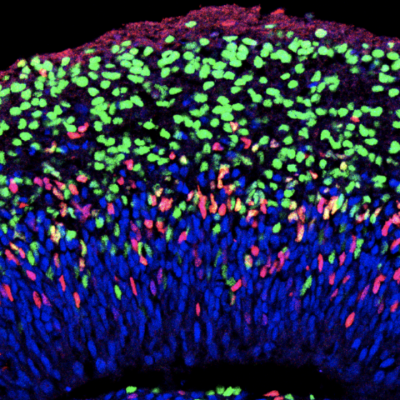
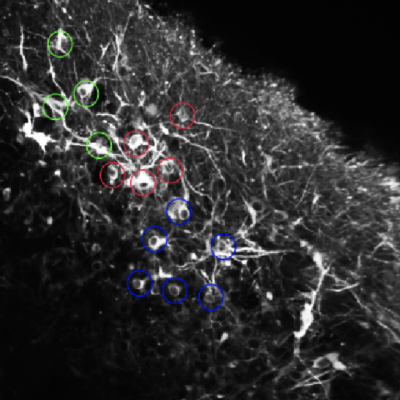
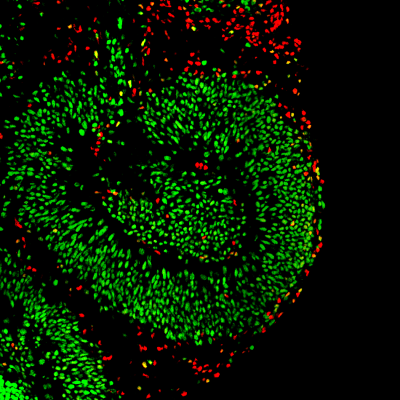
We seek to understand the biological mechanisms that result in the formation of neural circuits and how neurological disease can result in circuit dysfunction. Our goal is to apply a deeper understanding of neural circuit formation and function into improved treatments for some of the most devastating, and currently untreatable, brain diseases. To achieve this, we employ multiple cutting-edge approaches including novel 3D human stem cell-based in vitro structures called brain organoids, functional multiphoton and fluorescence lifetime imaging, advanced computational neuroscience techniques, electrophysiology, and transcriptomics.